I had the opportunity to join the Infectious Disease Association of California’s (IDAC) Spring 2025 Symposium this weekend. There was something both humbling and energizing about sitting in a room full of infectious disease experts, where the baseline knowledge was sky-high and my imposter syndrome was practically airborne. Rather than asking them to turn on the negative pressure and hand out N95s, I sharpened my focus and gleaned what I could from their brilliant ID minds – the kind of clinical nuance you don’t always get from just reading the literature.
This post is for those who couldn’t attend and want a condensed preview — filtered through one ID nurse practitioner’s scribbled notes and opinions. Not comprehensive. Not consensus. But hopefully, useful.
DISCLAIMER
This post reflects my personal insights and takeaways from the IDAC Spring 2025 Symposium. It’s not a comprehensive recap of each lecture, nor a substitute for the full content or speaker intent. I’ve done my best to highlight what stood out to me, supported by available references where appropriate — but as always, clinical decisions should be guided by primary literature, patient context, and sound judgment.
The symposium covered a wide range of topics — some highly practical, others refreshingly strange. Here’s the full lineup of lectures, marking the ones I’ll touch on below:
- C. diff Update
- Metagenomic Next Generation Sequencing: Diagnosis and Treatment
- Journal Club: Focus on Cross-Discipline Debates
- HIV Update
- Fascinating Local Cases
- The Oral Cephalosporins: Caveat Emptor
- Treatment and Prevention of Cardiovascular Implantable Electronic Device (CIED) Infections
- “Flu-Nami”
- Recurring Myths and Common Mistakes in the Management of Aspiration Pneumonia
C. diff Update
This fantastic talk given by Dr. Carl Crawford began with a review of microbiota-mediated colonization resistance — how a healthy gut flora protects against C. diff by regulating bile acid metabolism. Primary bile acids promote germination of C. diff spores, while secondary bile acids inhibit them.1 This balance gets disrupted by antibiotics, which explains why cumulative antibiotic exposure (more days, higher dose, more classes) dramatically increases C. diff risk. 2 Proton pump inhibitors were flagged as another contributor,3 with a side note that emerging PCABs may carry even more risk.
The speaker reviewed diagnostic tools — NAAT, GDH, EIA, and multistep algorithms — but the takeaway was: diagnostic clarity matters.
Treatment choice also plays a key role in adequate management. That brings us to fidaxomicin. As they put it:
“Fidaxomicin for your mother, vancomycin for your mother-in-law.”
Harsh, but the pharmacodynamics speak for themselves. Fidaxomicin spares key members of the microbiome, leading to fewer relapses and more sustained clinical cures4. Vancomycin, while still effective, disrupts the organisms responsible for converting primary bile acids into secondary ones. After treatment, primary bile acids return first — creating a “window of vulnerability” where spores can germinate unchecked due to a lack of secondary bile acids.5 This is part of why recurrence is so common, and why taper/pulse regimens may help — not by eradicating infection, but by holding C. diff at bay while the microbiota has time to recover.6
For recurrent cases of CDI, the speaker touched on fecal microbiota transplantation (still effective), and live biotherapeutics like SER-109, which aim to re-establish bile acid metabolism and colonization resistance.7 There’s also a growing list of non-biotic options (see below image) in development — microbiota-derived peptides, bacteriophages, bile acid analogs, and small molecules — but none yet ready for primetime.
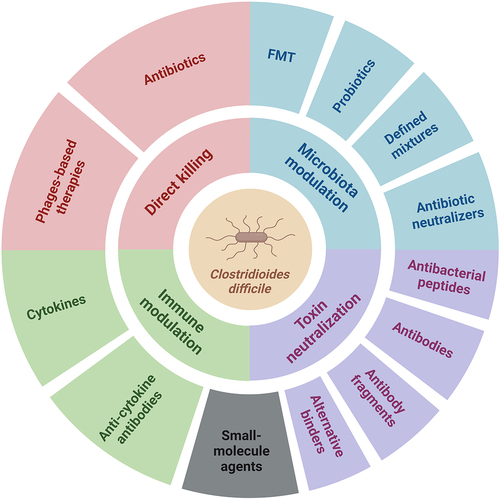
Journal Club: Two Highlights
This year’s Journal Club presented by Dr. Christopher Graber was a mix of cross-disciplinary curveballs and studies that made you pause mid-sip of hotel coffee. Two highlights stuck with me —
Rifaximin and the Daptomycin Domino Effect
This was a discussion about how rifaximin can drive daptomycin resistance in VRE. Specifically, rifaximin exposure selects for mutations in the RNA polymerase of VREfm, which leads to upregulation of the prdRAB operon. The result? Cell membrane remodeling that reduces daptomycin binding, creating a clinically significant and globally distributed mechanism of cross-resistance.8
This isn’t theoretical — these mutations have already been identified in circulating VREfm strains worldwide. The kicker: rifaximin has long been considered low-risk for resistance development. But in light of this, its widespread use in patients with cirrhosis may be compromising one of our last-resort therapies against multidrug-resistant pathogens.
So the next time you glance past rifaximin on a med list, pause. Your daptomycin MICs might depend on it.
Ertapenem in Hidradenitis Suppurativa
The second curveball came from dermatology — specifically, the use of ertapenem for 16 weeks for treatment of hidradenitis suppurativa (HS).9 Some derm groups are using it for its broad anaerobic coverage and potential anti-inflammatory effect, and in this series, patients reported meaningful improvements in both clinical symptoms and quality of life.10 Still, the evidence is early, and larger studies are needed to confirm whether it’s truly a game-changer or just a temporary reprieve.
For now, ertapenem may serve as a bridge to surgery in severe cases — but it definitely raises eyebrows. And maybe a few heart rates. Because nothing rattles an ID consult team like getting called for approval on a 16-week course of IV ertapenem… for acne-adjacent disease.
The Oral Cephalosporins
Jim Lewis, PharmD and co-chair of the breakpoint working group of the CLSI, didn’t mince words when it came to his stance on oral cephalosporins: he loathes them. The main issue? We’re probably underdosing them. These agents often fail to achieve the necessary T>MIC, meaning their clinical efficacy may not match what we expect from IV equivalents.
Not to mention, not all oral cephalosporins are created equal. To drive the point home, he included a visual that got a laugh —

Both are technically “dinosaurs,” but one is a child’s plush companion and the other, “will ruin your day in Jurassic Park.” The same applies to oral cephalosporins: grouping them together just because they share a name is lazy pharmacology.
(Credit to Jim Lewis, PharmD, for the analogy — and the dinosaur scare tactic.)
He spent time walking through several commonly used oral cephalosporins, showing how standard dosing often falls short of the PK/PD targets we aim for — even against something as simple as E. coli. Cephalexin, for example, likely requires 1 gram four times daily to achieve adequate target above MIC and should only be applied to non-severe, uncomplicated infections. Among the options, he noted that cefadroxil 1 gram every 6-8 hours may be the most reliable, offering a longer half-life and more predictable exposure.
The takeaway: be careful with your oral cephalosporins outside of urine sources and mild skin and soft tissue infections. Oral cephalosporins shouldn’t be your go-to unless you understand their PK/PD limitations, and “step-down” therapy isn’t always a lateral move. Sometimes, it’s a nosedive.
Aspiration Pneumonia: Myths That Refuse to Die
One of the more refreshing sessions by Dr. Ellie Goldstein tackled a common reflex in clinical medicine: automatically adding metronidazole or broad anaerobic coverage for suspected aspiration pneumonia. Historically, this reflex was built on studies like Goldstein et al. (1979), which demonstrated that anaerobes are frequently recovered in patients with necrotizing pneumonia, lung abscess, and putrid sputum.11
But in most acute, community-acquired aspiration pneumonias, anaerobes aren’t the primary pathogens. A 2019 NEJM review emphasized that the causative organisms usually mirror typical CAP flora — like Streptococcus pneumoniae, Staph aureus, and aerobic gram-negatives.12
Now, even if anaerobes are on your differential, don’t assume your standard empiric agents will cover them. A 2006 in vitro study showed that azithromycin has poor activity against oral anaerobes, and that if you’re truly concerned about anaerobes, agents like amoxicillin-clavulanate or clindamycin are more reliable.13
So what’s the verdict? Aspiration pneumonia is often just regular pneumonia in a slightly different location — and routine anaerobic coverage isn’t necessary unless the clinical context strongly supports it (e.g. severe periodontal disease, putrid sputum, lung abscess).
We’ve learned to fear anaerobes — and sometimes, rightfully so. But reflexively reaching for flagyl or zosyn every time someone coughs post-prandially might say more about our habits than the bugs we’re treating.
Well, there you have it — thirteen hours of lectures distilled into a handful of paragraphs. (Twenty-two, I think. But who’s counting?)
These takeaways are just the ones that stuck with me — filtered through one ID nurse practitioner’s intellect and personal biases. I hope they spark a question, reinforce a hunch, or make you pause before co-signing that next 16-week ertapenem order.
Thanks for coming along for the ride. See you at the next conference — I’ll be the one sitting quietly, pretending not to be panicking about whether I’m hitting T>MIC.
- Theriot CM, Young VB. Interactions Between the Gastrointestinal Microbiome and Clostridium difficile. Annu Rev Microbiol. 2015;69:445-61. doi: 10.1146/annurev-micro-091014-104115. PMID: 26488281; PMCID: PMC4892173. ↩︎
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011 Jul 1;53(1):42-8. doi: 10.1093/cid/cir301. PMID: 21653301. ↩︎
- Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010 May 10;170(9):784-90. doi: 10.1001/archinternmed.2010.89. PMID: 20458086. ↩︎
- Louie TJ et al. “Fidaxomicin versus vancomycin for Clostridium difficile infection.” NEJM 2011. [PMID: 21596671] ↩︎
- Qian X, Yanagi K, Kane AV, Alden N, Lei M, Snydman DR, Vickers RJ, Lee K, Thorpe CM. Ridinilazole, a narrow spectrum antibiotic for treatment of Clostridioides difficile infection, enhances preservation of microbiota-dependent bile acids. Am J Physiol Gastrointest Liver Physiol. 2020 Aug 1;319(2):G227-G237. doi: 10.1152/ajpgi.00046.2020. Epub 2020 Jun 29. PMID: 32597706; PMCID: PMC7500266.
↩︎ - Sehgal K, Zandvakili I, Tariq R, Pardi DS, Khanna S. Systematic Review and Meta-Analysis: Efficacy of Vancomycin Taper and Pulse Regimens in Clostridioides difficile Infection. Expert Rev Anti Infect Ther. 2022 Apr;20(4):577-583. doi: 10.1080/14787210.2022.1997588. Epub 2021 Nov 1. PMID: 34693838.
↩︎ - Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, Sims M, Kraft CS, Cohen SH, Berenson CS, Korman LY, Ford CB, Litcofsky KD, Lombardo MJ, Wortman JR, Wu H, Auniņš JG, McChalicher CWJ, Winkler JA, McGovern BH, Trucksis M, Henn MR, von Moltke L. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N Engl J Med. 2022 Jan 20;386(3):220-229. doi: 10.1056/NEJMoa2106516. PMID: 35045228. ↩︎
- Turner, A.M., Li, L., Monk, I.R. et al. Rifaximin prophylaxis causes resistance to the last-resort antibiotic daptomycin. Nature 635, 969–977 (2024). https://doi.org/10.1038/s41586-024-08095-4 ↩︎
- Nosrati A, Ch’en PY, Torpey ME, et al. Efficacy and Durability of Intravenous Ertapenem Therapy for Recalcitrant Hidradenitis Suppurativa. JAMA Dermatol. 2024;160(3):312–318. doi:10.1001/jamadermatol.2023.6201 ↩︎
- Join-Lambert O, Coignard-Biehler H, Jais J-P, et al. Ertapenem in severe hidradenitis suppurativa: A retrospective study of efficacy and safety. Br J Dermatol. 2016;174(1):70–76. PMID: 26332689 ↩︎
- Goldstein EJC, et al. Erythromycin for Anaerobic Pleuropulmonary and Soft-Tissue Infections. JAMA. 1979;242(5):435–438. ↩︎
- Mandell LA, Niederman MS. Aspiration Pneumonia. N Engl J Med. 2019;380(7):651–663. ↩︎
- Merriam CV, et al. In vitro activity of azithromycin and nine comparator agents against 296 strains of oral anaerobes. Int J Antimicrob Agents. 2006;28(3):244–248. ↩︎

Leave a comment